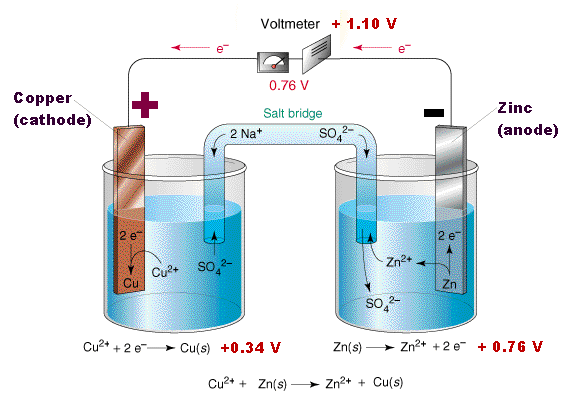
what is salt bridge?
explain its function?
Salt Bridge: A salt bridge is a U- shape containing concentrated solution of an inert electrolyte like KCl, KNO3, K2SO4 etc.or a solidified solution of those electrolytes in agar-agar solution and gelatin.
These inert electrolytes do not take part in redox reaction of the cell and even dont react with the electrolyte used.
Function: the main functions of a salt bridge are;
- They allow the movement of ions from one solution to another without mixing of the two solutions and complete the electrical circuit.
- To maintain the electical neutrality of the solutions in the two half cells.