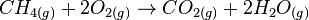what is combustion ??
Dear user!
Combustion can be defined as burning of any substance in presence of atmospheric oxygen. The following reaction shows a combustion reaction wherein methane is burnt in atmospheric oxygen to form carbon dioxide and water vapour.
CH4 + 2O2 → CO2 + 2H2O
Combustion is an exothermic process i.e., the process is accompanied with the evolution of heat. The release of heat may or may not result in the production of light. Thus, two important things required for the process of combustion to take place are a fuel (combustible substance) and supporter of combustion (oxygen).
I hope, with this the concept of combustion becomes clear.
All the best!!